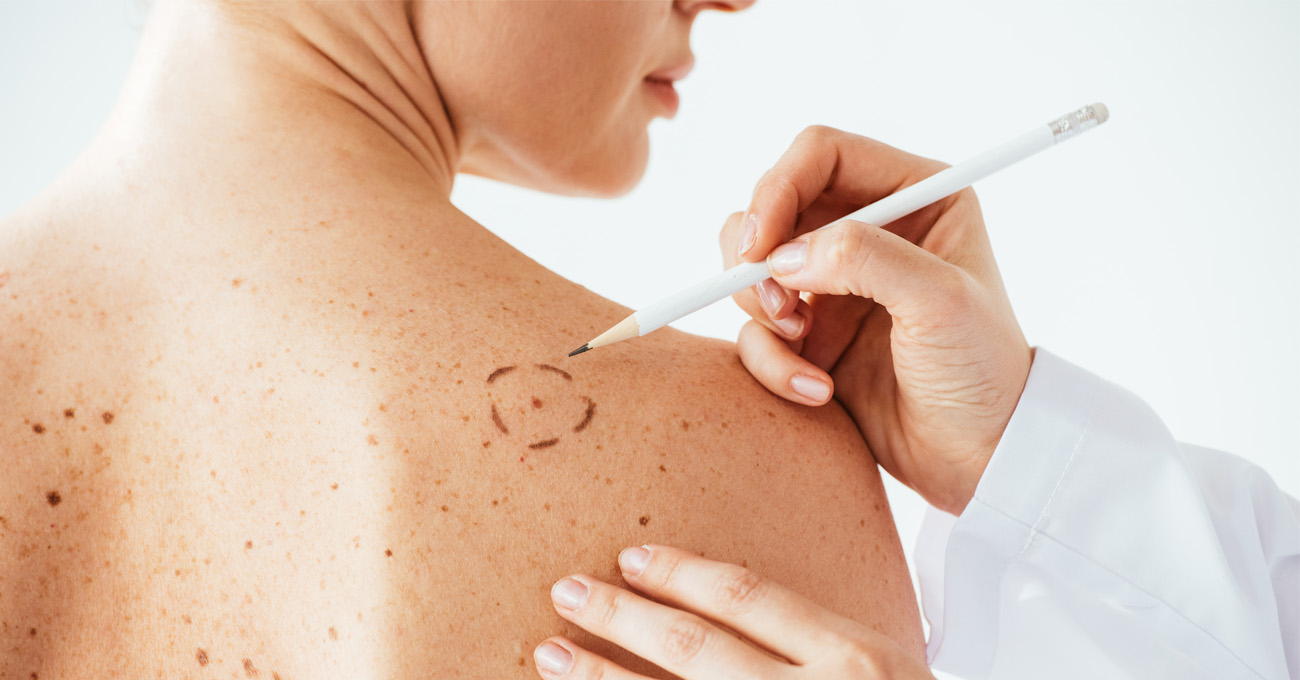
Advocate Aurora Health cancer clinics across Illinois and Wisconsin are the first in either state to join an international clinical trial that seeks to identify the ideal amount of skin to be removed from around a melanoma, the most serious type of skin cancer.
“The typical treatment for a melanoma is surgery to remove the tumor,” said oncologist Sigrun Hallmeyer, MD, Advocate Aurora Research Institute’s principal investigator for the study. “After surgery, doctors analyze the tumor’s thickness to determine a treatment plan and then often perform a second surgery to remove a larger amount of skin from around the initial excision. However, there remains disagreement about how large of a margin of healthy tissue to remove.”
Current clinical guidelines advise removing 1 to 3 cm of healthy skin around the melanoma. Such a wide excision can leave a rather sizable wound from which the patient needs to heal.
In this clinical trial, which is being conducted across eight countries, researchers are looking to determine if a 1-cm margin is as good as a 2-cm margin at reducing the risk of melanoma returning. They are also investigating whether a margin that is less than 2 cm causes fewer side effects and improves quality of life.
“Results from previous studies evaluating ideal melanoma margins have been inconclusive,” said Nina Garlie, PhD, vice president of clinical trials research for the Research Institute. “Meanwhile, there is a growing concern among doctors that larger excisions are leading to longer hospital stays and rehabilitation, and a greater risk for chronic pain or even loss of function.”
Wider excisions are also more disfiguring for patients with melanomas, which often appear on skin that is frequently exposed to the sun and therefore visible to others, such as the arms, legs and face.
“If this trial shows that a 1-cm margin is sufficient, future patients will be spared unnecessary and cosmetically more challenging procedures,” Dr. Hallmeyer said.
Participants will be randomly assigned to have either a 1-cm or a 2-cm margin of healthy tissue removed during surgery. Researchers will then follow participants’ conditions for five years.
Advocate Aurora is participating in the study through its inclusion in the National Cancer Institute’s (NCI) Community Oncology Research Program (NCORP), which brings cancer clinical trials to people in their own communities instead of only at major research institutions. This clinical trial is being conducted at all 29 of Advocate Aurora NCORP sites.
Researchers plan to enroll about 3,000 participants from around the world in the study, “Melanoma margins trial investigating 1cm v 2cm wide excision margins for primary cutaneous melanoma (MelMarT-II).” In the U.S., the trial is coordinated by SWOG Cancer Research Network, a cooperative research group that designs and conducts clinical trials under the sponsorship of NCI. Melanoma and Skin Cancer Trials, based in Australia, is the global sponsor for the trial.
To learn more about Advocate Aurora’s research, visit aah.org/research.